Throughout human history, milk has been revered for its remarkable nutritional content. High in quality proteins, vitamins, minerals, and fats, milk offers a complete package of essential nutrients. A predominant feature of milk’s nutritional profile is its protein content, specifically the presence of casein and whey proteins. In this article, we will traverse the fascinating journey of these milk proteins, starting from their processing, exploring their behaviour in the stomach and absorption, and ending with their significant role in muscle protein synthesis.
Milk Proteins: a nutritional powerhouse
Milk’s proteins are primarily casein, accounting for nearly 80%, and whey proteins, making up the remaining 20%. Each protein type brings unique characteristics to the table, leading to their classification as ‘slow-digesting’ (casein) and ‘fast-digesting’ (whey) proteins. This distinction has important implications for our health and wellbeing, shaping how our bodies utilise these proteins post-consumption1.
Processing of milk proteins
Even before gracing our tables, milk proteins commence a transformative journey through various stages of processing. This journey shapes their digestibility, nutrient profile, and the ultimate impact they have on our health.
Heat treatment, a procedure commonly employed during milk processing, brings about significant changes in milk proteins’ structure. This crucial step has a profound impact on how our body interacts with and derives nutrition from these proteins. When exposed to heat, proteins undergo changes at a molecular level – some form new bonds, a phenomenon known as ‘cross-linking’, which makes them more resilient to digestion. On the other hand, some proteins denature or unfold, which enhances their susceptibility to enzymatic degradation in our bodies1.
The interplay between casein and whey, the two primary types of milk proteins, is also impacted during processing. Heat treatment alters their coagulation properties, influencing how they behave once inside our stomach. Casein proteins, for instance, have a natural propensity to aggregate and form a gel-like structure in acidic conditions, such as the stomach. This process results in a slow and steady release of amino acids during digestion. However, certain processing methods, like the conversion to caseinate or the formation of stirred yogurts, can temper this coagulation, thereby accelerating the digestion and absorption of casein proteins1.
Meanwhile, whey proteins, due to their soluble nature, digest swiftly and lead to a quick surge of amino acids in our bloodstream. This swift absorption, combined with their high leucine content – an essential amino acid vital for muscle protein synthesis – has led to the popular use of whey proteins in dietary supplements1.
The stomach’s role: gastric coagulation
Upon consumption, milk proteins embark on a fascinating transformation within our stomach. Gastric coagulation, as this process is known, plays a key role in determining how our bodies make use of these proteins. The stomach’s acidic environment causes casein proteins to aggregate, forming a ‘coagulum’ or a ‘curd’. This curd-like structure then slowly releases amino acids, allowing for a steady, extended absorption period1.
Contrarily, whey proteins do not form a coagulum. They retain a liquid state and are quickly evacuated from the stomach, leading to their constituent amino acids’ rapid absorption. This difference in behaviour results in a faster uptake of amino acids from whey proteins compared to casein1.
It is important to note that the processing methods applied to milk proteins influence this gastric coagulation phenomenon. For instance, certain manufacturing processes can modify the structure of casein, making it more resistant to coagulation and altering the kinetics of amino acid release. Similarly, the composition and structure of dairy products, such as yogurt or cheese, can impact gastric coagulation and subsequent amino acid absorption. Factors like moisture content, fat content, calcium content, and the density of the product all contribute to the intricate interplay between protein digestion, gastric coagulation, and amino acid availability1.
The rise of amino acids: postprandial aminoacidemia
The varied speed of digestion and absorption of these proteins gives rise to a phenomenon known as ‘postprandial aminoacidemia’. This term refers to the surge in blood amino acid levels following protein intake. Dietary proteins are broken down into amino acids, their basic building blocks, which are then absorbed into the bloodstream. This rise in available amino acids fuels the function and regeneration of various body tissues, such as skeletal muscles1.
Around half of the dietary protein we consume is effectively digested and absorbed, becoming available for use by peripheral tissues. The remaining portion is utilised by splanchnic tissues like the gut and liver. This influx of amino acids into the bloodstream triggers the release of insulin, enhances tissue perfusion, and initiates the process of tissue protein synthesis1.
Muscle health and milk proteins: a synergistic relationship
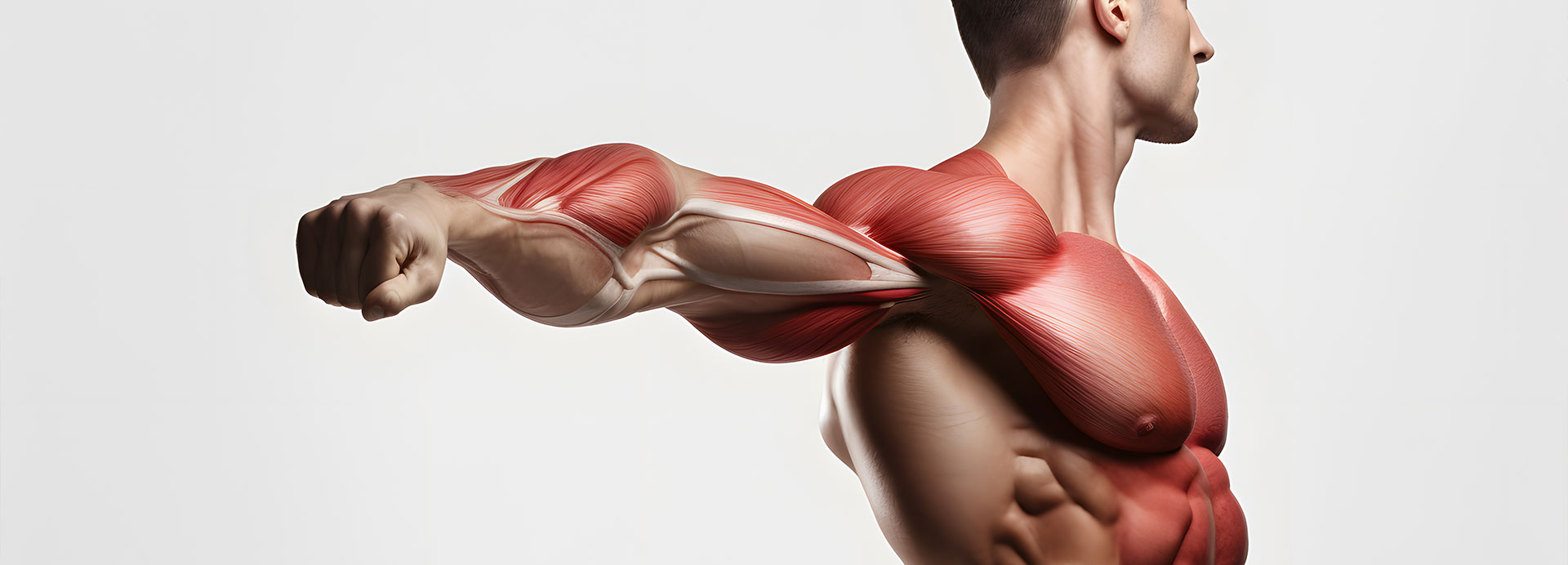
The muscle tissue, one of the main beneficiaries of this postprandial amino acid surge, is a focus of extensive research. Muscle tissue is in a constant state of renewal, and the maintenance of muscle mass depends on a delicate balance between muscle protein synthesis and breakdown. Dietary protein, particularly from milk, plays a crucial role in this balance1.
Ingestion of milk proteins provides the necessary amino acids for muscle tissue repair and growth. High-quality milk proteins, rich in essential amino acids and especially leucine, stimulate muscle protein synthesis and reduce muscle protein breakdown, thereby promoting muscle health1.
Increasing amino acid concentrations in the blood do not always result in higher muscle protein synthesis rates. This inconsistency may stem from ‘anabolic resistance’, a phenomenon more common in aging and inactivity, where muscle protein synthesis response to dietary protein is reduced. However, if protein intake is sub-optimal (less than 20g), a heightened postprandial plasma amino acid response might stimulate muscle protein synthesis more effectively1.
These findings emphasize the importance of tailored nutritional strategies that account for individual circumstances and requirements. While higher amino acid responses do not necessarily equate to elevated muscle protein synthesis rates in healthy, active individuals, certain conditions warrant special attention. In cases where anabolic resistance poses a challenge, or when protein intake is sub-optimal, maximizing the postprandial amino acid surge becomes critical for supporting muscle protein synthesis and preserving muscle mass1.
Muscle maintenance during lifetime:
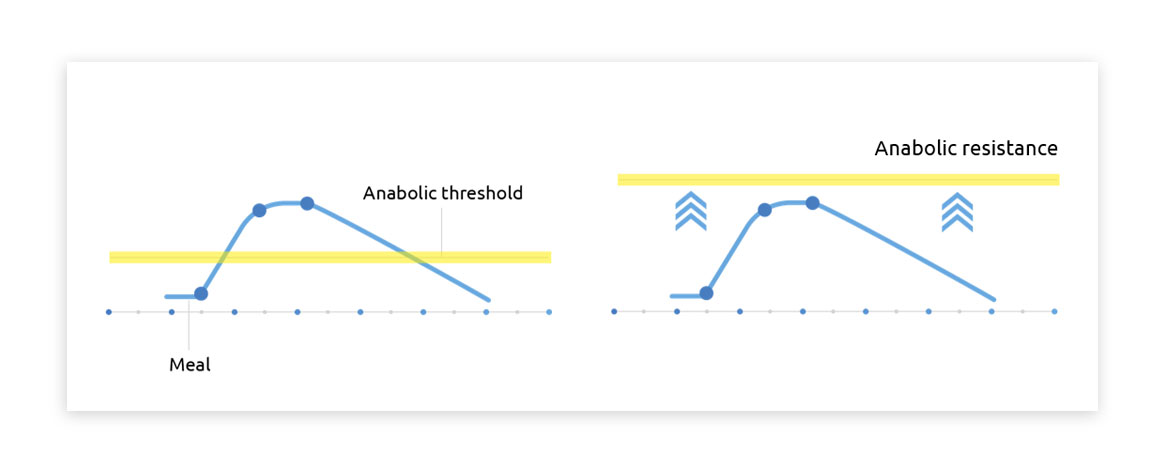
Maintenance of muscle mass:
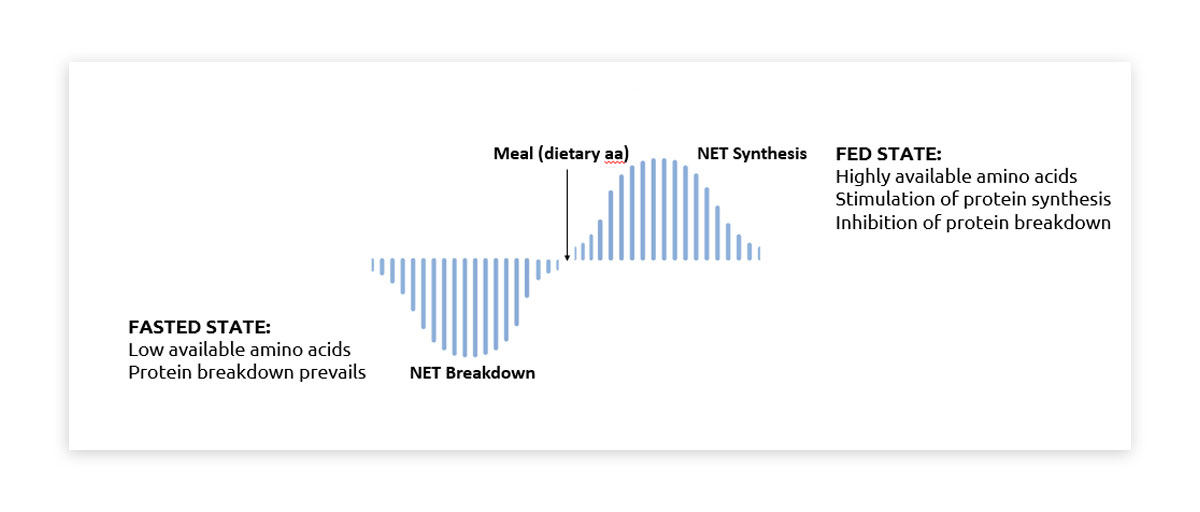
From processing to muscle protein synthesis
Our understanding of milk proteins has come a long way since research began in the early 19th century. It’s fascinating to trace the journey from the coagulation of milk proteins in the stomach to their role in stimulating muscle protein synthesis.
The key to harnessing the benefits of milk proteins lies in understanding how processing, food structure, and preservation can influence the digestion and absorption of these proteins. Tailoring these factors to individual needs could prove essential, particularly for those who need an optimal amino acid response for maintaining or increasing muscle mass. In essence, the power of milk proteins extends beyond the glass, reaching into the core of our physiology, and contributing to our health and wellbeing.
References: